Weak Diprotic Acid Titration Curve
How to Sketch Titration Bend for Diprotic Acid
In this JC2 webinar we want to larn how to sketch titration curve for diprotic acrid.
Let's consider the titration of ethanedioic acrid (H2C2O4) with sodium hydroxide.
A diprotic acrid will react with OH- in two stages:
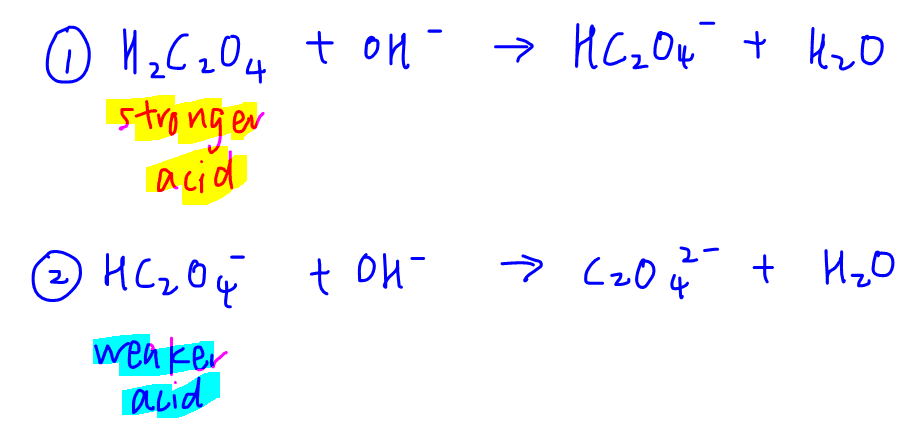
Each stage can be treated as a monoprotic acid-base of operations reaction.
First acid H2C2Oiv is stronger than second acid HC2O4 -, hence reaction 1 will go along first and go to completion before the second reaction volition outset.
At the end of each reaction, there will be an equivalence signal.
Therefore we accept 2 distinct reactions with 2 distinct equivalence points.
Here are the 3 points that we tin can apply for plotting:
1. Initial pH
ii. Maximum buffering capacity during reaction 1
iii. Maximum buffering chapters during reaction ii
one. Initial pH
For a diprotic acrid, we presume that the H+ only comes from the first dissociation of H2C2O4.
The second acid HCtwoO4 - is weaker and volition contribute an insignificant corporeality of H+.
So we tin can only care for a diprotic acrid like a monoprotic acid, and focus on its first dissociation.
Hence the weak acrid formula can be used to determine H+ concentration and pH.
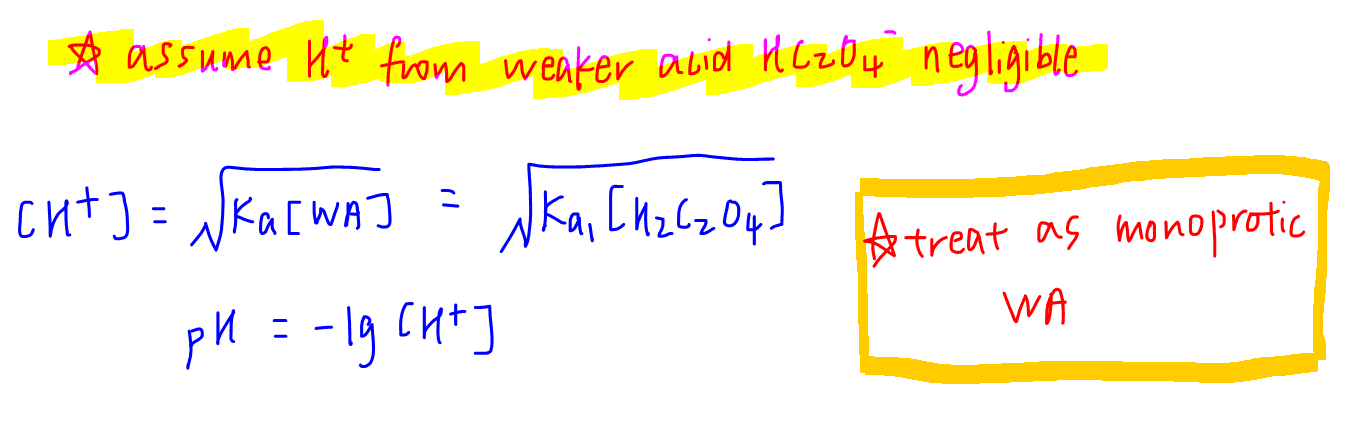
2. Maximum buffering capacity during reaction 1
During reaction 1, H2C2O4 is in backlog and a buffer is formed.
This buffering system is made up of weak acrid H2C2O4 and cohabit base HC2Ofour -.
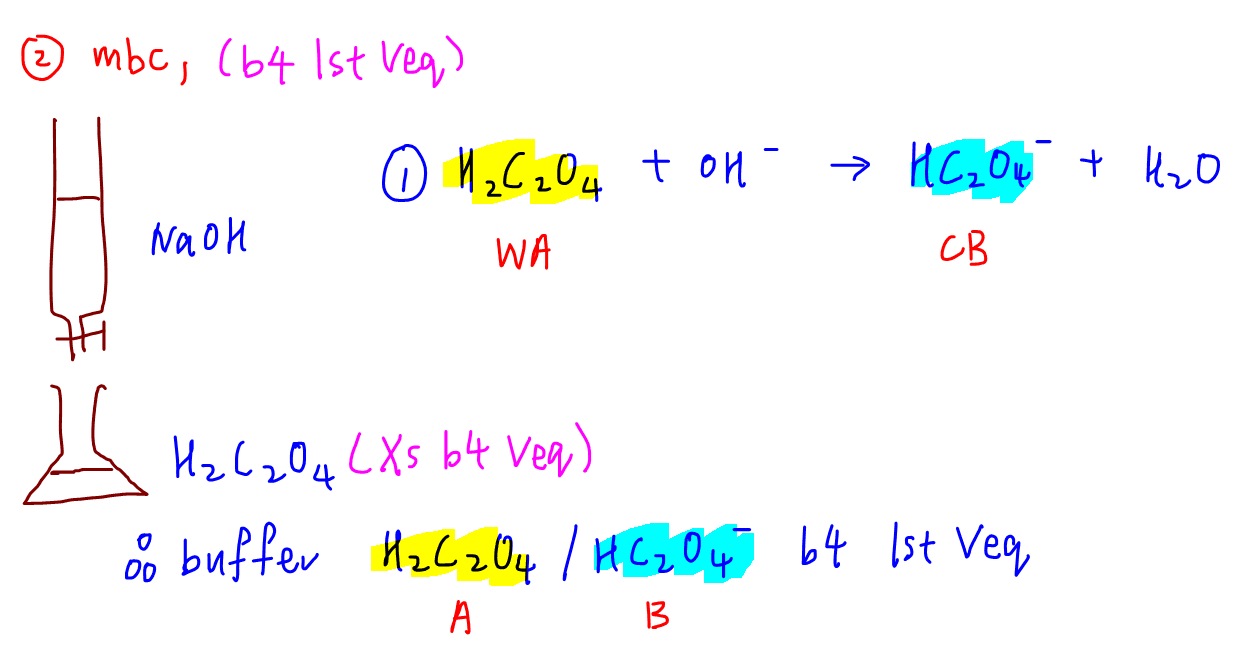
Hence we can determine its maximum buffering capacity for the commencement buffer region.

Mbc is very useful to plot on a titration curve since gradient at that point is zero.
Therefore the shape of the graph at and effectually mbc will exist very well defined.
3. Maximum buffering capacity during reaction 2
Similarly, during reaction two, HC2O4 - is in excess and a buffer is formed.
This buffering arrangement is fabricated up of weak acid HC2Ofour - and conjugate base C2O4 two-.

Hence we tin can determine its maximum buffering capacity for the second buffer region.

Let'southward put everything together and plot our titration curve!
We tin can make employ of the maximum buffering capacities to depict the buffer regions for both reactions.
The range of rapid pH changes at both equivalence points tin then exist added.

With these 3 points we tin can sketch a very decent looking titration curve for diprotic acids.
Topic: Buffer and Titration Curve, Physical Chemistry, A Level Chemical science, Singapore
Back to other previous Physical Chemistry Video Lessons.
Found this A Level Chemistry video useful?
Please Like this video and SHARE it with your friends!
Join my 7000+ subscribers on myYouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Demand an experienced tutor to make Chemical science simpler for you?
Do consider signing upwardly for my JC Chemistry Tuition classes at Bishan, weekly LIVE webinars or on-demand video lessons!
Weak Diprotic Acid Titration Curve,
Source: https://chemistryguru.com.sg/diprotic-acid-titration-curve
Posted by: godwinaces1963.blogspot.com


0 Response to "Weak Diprotic Acid Titration Curve"
Post a Comment